The Role of Graphene’s Electrical Conductivity in Enhancing Hydrogen Catalytic Decomposition
Hydrogen is a key player in the transition to sustainable energy systems, and its efficient production and utilization depend heavily on catalytic processes. Among these, the catalytic decomposition of hydrogen molecules is crucial for applications such as hydrogen storage, fuel cells, and other hydrogen energy technologies. Graphene, a two-dimensional carbon material with remarkable electrical conductivity, has garnered attention for its ability to enhance catalytic reactions, including hydrogen decomposition.
This article explores how graphene’s superior electrical conductivity contributes to improved catalytic decomposition of hydrogen, examining its mechanisms, material innovations, and potential applications.
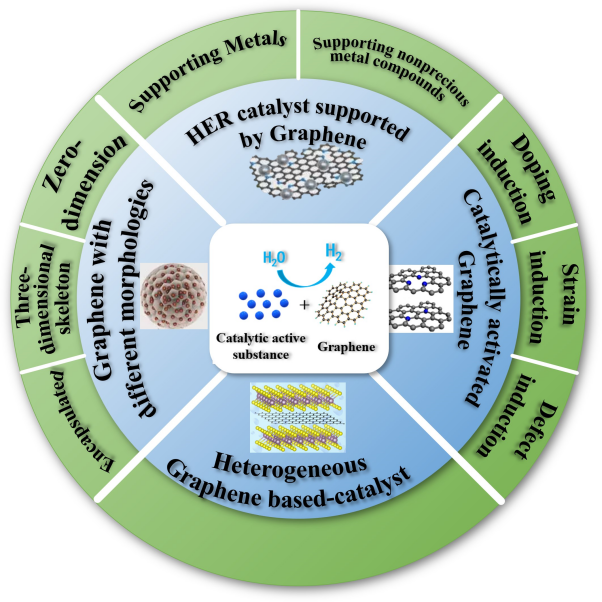
Key Properties of Graphene in Catalysis
Graphene possesses several intrinsic properties that make it advantageous in catalytic systems:
- Exceptional Electrical Conductivity:
- Facilitates rapid electron transfer during catalytic reactions, reducing activation energy.
- High Surface Area:
- Provides abundant active sites for catalysts to interact with hydrogen molecules.
- Mechanical and Thermal Stability:
- Ensures durability under reaction conditions involving high temperatures or mechanical stress.
- Chemical Tunability:
- Functional groups or doping with heteroatoms can improve graphene’s catalytic activity.
Mechanism of Hydrogen Catalytic Decomposition on Graphene
The decomposition of hydrogen molecules (H2H_2) involves breaking the H−HH-H bond and dissociating the molecule into individual hydrogen atoms. Graphene enhances this process in several ways:
- Electron Transfer:
- Graphene’s high conductivity enables efficient electron transfer between the hydrogen molecule and the catalytic sites. This lowers the energy barrier for dissociation.
- Support for Catalytic Nanoparticles:
- Graphene serves as an ideal substrate for metal catalysts (e.g., platinum, palladium, or nickel), stabilizing the nanoparticles and preventing aggregation while ensuring uniform electron flow.
- Spillover Effect:
- Graphene promotes the migration of dissociated hydrogen atoms from the catalyst to its surface, increasing overall adsorption capacity.
- Defect Engineering:
- Structural defects in graphene, such as vacancies or edges, act as active sites for hydrogen molecule adsorption and dissociation.
Enhancing Catalytic Activity Through Graphene Conductivity
- Metal-Graphene Composites:
- Combining graphene with transition metals significantly enhances catalytic efficiency. For instance, platinum nanoparticles on graphene have shown higher activity in hydrogen decomposition due to improved charge transfer.
- Doped Graphene:
- Nitrogen-doped graphene alters electronic properties, introducing localized electron density favorable for hydrogen activation.
- Hybrid Materials:
- Integrating graphene with materials like metal-organic frameworks (MOFs) or carbon nanotubes (CNTs) creates hybrid structures that leverage graphene’s conductivity for enhanced hydrogen catalysis.
- Plasma-Modified Graphene:
- Plasma treatments introduce functional groups or defects that increase graphene’s catalytic activity while maintaining its high conductivity.
Applications of Graphene in Hydrogen Catalysis
- Hydrogen Production:
- In water-splitting reactions, graphene-based catalysts enhance the hydrogen evolution reaction (HER) by facilitating electron flow to catalytic sites.
- Hydrogen Fuel Cells:
- Graphene improves the performance of proton-exchange membrane fuel cells (PEMFCs) by supporting efficient hydrogen oxidation at the anode.
- Onboard Hydrogen Storage Systems:
- Catalysts supported on graphene can aid in the rapid release of hydrogen from storage materials, such as metal hydrides, via catalytic decomposition.
- Hydrogen Sensors:
- Graphene’s conductivity changes in the presence of hydrogen, enabling the design of sensitive and efficient hydrogen detection systems.
Case Studies and Experimental Evidence
- Platinum on Graphene Substrates:
- Studies demonstrate that graphene-supported platinum nanoparticles exhibit a 2–3 times higher catalytic activity for hydrogen decomposition compared to traditional supports like carbon black.
- Nitrogen-Doped Graphene:
- Research has shown that nitrogen doping increases the charge density around catalytic sites, reducing the activation energy for H2H_2 dissociation by up to 40%.
- Graphene-Palladium Composites:
- In experiments, palladium nanoparticles on graphene exhibited enhanced hydrogen adsorption and decomposition, attributed to graphene’s high conductivity and stability.
Challenges and Future Directions
- Scalability of High-Conductivity Graphene:
- Producing graphene with consistently high conductivity on a large scale remains a challenge.
- Optimizing Catalyst-Graphene Interactions:
- Ensuring uniform distribution and strong interaction between metal nanoparticles and graphene is critical for achieving peak performance.
- Cost Reduction:
- The high cost of noble metals like platinum necessitates research into alternative catalysts that leverage graphene’s conductivity.
- Durability in Harsh Conditions:
- Prolonged operation in high-temperature or corrosive environments requires graphene-based catalysts to maintain their conductivity and structural integrity.
Conclusion
Graphene’s unparalleled electrical conductivity plays a pivotal role in enhancing the catalytic decomposition of hydrogen. By facilitating rapid electron transfer, stabilizing catalytic nanoparticles, and supporting the spillover effect, graphene-based systems demonstrate superior performance in hydrogen-related technologies.
Advancements in graphene engineering—such as doping, hybridization, and defect introduction—offer pathways to further optimize its catalytic properties. As research continues to overcome challenges in scalability and cost, graphene’s role in hydrogen energy systems is poised to expand, driving innovations in production, storage, and utilization of clean energy.

