Graphene Nitride: Production, Properties, and Applications
Graphene nitride, also known as nitrogen-doped graphene (NG), is a graphene derivative that incorporates nitrogen atoms into its carbon lattice. The introduction of nitrogen alters graphene’s electronic structure and enhances its properties, making it suitable for advanced applications in catalysis, energy storage, and sensing. In this article, we explore the synthesis methods, advantages, and industrial applications of graphene nitride.
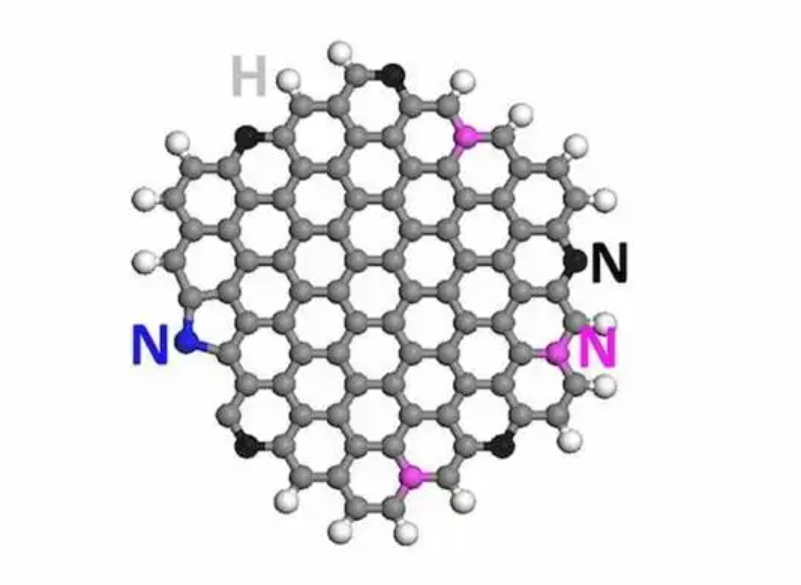
1. What is Graphene Nitride?
Graphene nitride is a modified form of graphene in which nitrogen atoms are substituted into the carbon lattice. This doping process creates new active sites and modulates the electronic properties of graphene, making it more versatile than pristine graphene.
Nitrogen atoms can exist in the graphene lattice in different forms, including pyridinic, pyrrolic, and graphitic nitrogen, each contributing differently to the material’s properties. These forms impact the electrical conductivity, catalytic activity, and chemical stability of graphene nitride.
2. Synthesis Methods of Graphene Nitride
The production of graphene nitride involves introducing nitrogen atoms into the graphene structure through a variety of methods. The choice of method affects the type and concentration of nitrogen doping and the resulting material properties.
2.1 Chemical Vapor Deposition (CVD)
Chemical vapor deposition is a common technique for synthesizing graphene nitride. This method involves exposing graphene or a carbon source to a nitrogen-containing gas (such as ammonia) at high temperatures.
- Process: A substrate coated with graphene or a carbon precursor is placed in a furnace. Ammonia or other nitrogen-rich gases are introduced, and nitrogen atoms are incorporated into the carbon lattice during the reaction.
- Advantages: CVD enables precise control over nitrogen content and doping uniformity, resulting in high-quality graphene nitride.
2.2 Thermal Annealing
Thermal annealing involves treating graphene oxide (GO) or reduced graphene oxide (rGO) in the presence of nitrogen-rich compounds, such as urea or ammonia, at elevated temperatures.
- Process: GO is dispersed in a solution containing the nitrogen source and then subjected to high-temperature annealing.
- Advantages: This method is cost-effective and scalable, making it suitable for large-scale production.
2.3 Hydrothermal Synthesis
Hydrothermal synthesis uses a nitrogen precursor in an aqueous environment under high temperature and pressure to achieve nitrogen doping.
- Process: GO is mixed with a nitrogen source, such as hydrazine or ammonium hydroxide, and heated in a sealed autoclave.
- Advantages: This method is eco-friendly and allows for the simultaneous reduction and doping of GO.
2.4 Plasma Treatment
In plasma treatment, graphene or GO is exposed to a nitrogen plasma environment, which facilitates the direct incorporation of nitrogen atoms into the lattice.
- Advantages: Plasma treatment is a surface-specific technique, enabling targeted doping and modification of graphene surfaces.
3. Advantages of Graphene Nitride
Graphene nitride offers a range of enhanced properties that stem from the incorporation of nitrogen atoms into its lattice:
3.1 Improved Conductivity
Nitrogen doping modulates the electronic band structure of graphene, improving its electrical conductivity. This is particularly beneficial for applications requiring rapid electron transfer, such as batteries and supercapacitors.
3.2 Enhanced Catalytic Performance
The active sites created by nitrogen doping enhance graphene’s catalytic properties, making it suitable for use as an electrocatalyst in reactions like hydrogen evolution and oxygen reduction.
3.3 Increased Corrosion Resistance
Graphene nitride exhibits improved chemical stability and resistance to oxidation, extending its lifespan in harsh environments.
3.4 Greater Stability
The incorporation of nitrogen atoms enhances the thermal and mechanical stability of graphene, broadening its application range.
4. Applications of Graphene Nitride
The unique properties of graphene nitride enable its use in a wide range of industries, particularly in energy storage, catalysis, and sensing.
4.1 Electrocatalysis
One of the most significant applications of graphene nitride is in electrocatalysis, where it serves as a catalyst for key reactions:
- Hydrogen Evolution Reaction (HER): In water splitting, graphene nitride acts as an electrocatalyst to produce hydrogen, an essential component of clean energy systems.
- Oxygen Reduction Reaction (ORR): In fuel cells, graphene nitride catalyzes the reduction of oxygen, improving efficiency and performance.
The active nitrogen sites in graphene nitride make it a viable, cost-effective alternative to expensive precious metal catalysts like platinum.
4.2 Energy Storage: Batteries and Supercapacitors
Graphene nitride improves the efficiency of energy storage devices by facilitating faster charge transfer and enhancing storage capacity.
- Lithium-Ion Batteries: Used as an electrode material, graphene nitride improves battery performance by increasing energy density and cycle stability.
- Supercapacitors: Its large surface area and excellent conductivity allow graphene nitride to store and release energy rapidly, making it ideal for high-power applications.
4.3 Sensors
Graphene nitride is highly sensitive to environmental changes, making it an excellent material for sensors.
- Gas Sensors: It can detect gases such as ammonia, nitrogen dioxide, and hydrogen with high precision, thanks to the active sites created by nitrogen doping.
- Chemical Sensors: The functionalized surface of graphene nitride enables the detection of specific chemicals, broadening its use in environmental monitoring and safety.
4.4 Advanced Composites
Graphene nitride can be incorporated into composites to improve their properties:
- Structural Composites: Used in aerospace and automotive industries, it enhances mechanical strength and reduces weight.
- Anti-Corrosion Coatings: The improved corrosion resistance of graphene nitride makes it ideal for protective coatings in harsh environments.
5. Challenges and Future Directions
While graphene nitride has demonstrated immense potential, certain challenges must be addressed to fully exploit its capabilities:
- Scalability: Many synthesis methods are still limited in scalability, and consistent quality in large-scale production remains a challenge.
- Optimization: Achieving precise control over the type and distribution of nitrogen doping is critical for tailored applications.
- Cost: Reduction of production costs is necessary for widespread commercial adoption.
Future research will likely focus on developing greener, more efficient synthesis methods and exploring new applications for graphene nitride in emerging technologies like quantum computing, biomedicine, and sustainable energy systems.
6. Conclusion
Graphene nitride represents a significant advancement in the field of graphene-based materials, combining the versatility of graphene with enhanced conductivity, catalytic activity, and stability. Its applications in electrocatalysis, energy storage, sensing, and advanced composites position it as a cornerstone material for next-generation technologies.
As research and development continue, graphene nitride is poised to play a transformative role in addressing global challenges in energy, environment, and industry. With its exceptional properties and growing potential, graphene nitride exemplifies the promise of nanotechnology in shaping a sustainable future.

