Graphene Explained: Structure, Properties, Applications & Future Trends
What is Graphene?
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice. It is the thinnest, strongest, and one of the most conductive materials ever discovered. Since its first isolation in 2004 by Andre Geim and Konstantin Novoselov, who later won the Nobel Prize in Physics (2010), graphene has attracted tremendous attention across industries—from electronics to energy.
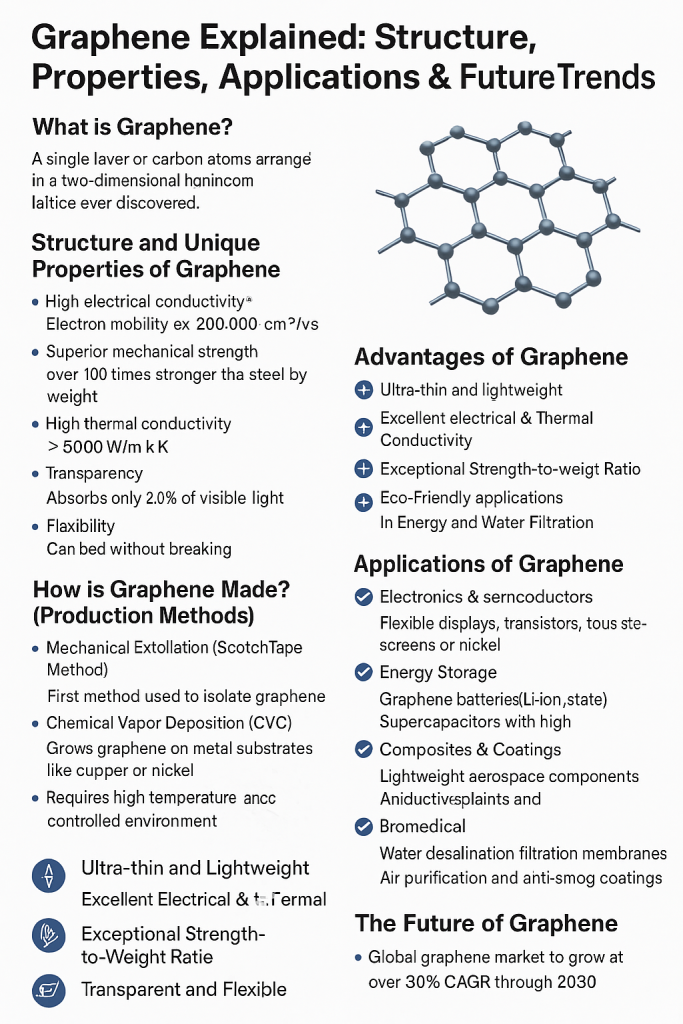
Structure and Unique Properties of Graphene
Graphene’s structure is incredibly simple yet profoundly powerful. Each carbon atom forms strong covalent bonds with three neighboring atoms, creating a flat, hexagonal network only one atom thick. Despite its atomic thinness, graphene exhibits:
-
High electrical conductivity — Electron mobility exceeds 200,000 cm²/V·s.
-
Superior mechanical strength — Over 100× stronger than steel by weight.
-
High thermal conductivity — >5000 W/m·K.
-
Transparency — Absorbs only ~2.3% of visible light.
-
Flexibility — Can bend without breaking, ideal for flexible electronics.
These remarkable traits make graphene a game-changer in both research and commercialization.
How is Graphene Made? (Production Methods)
There are several methods to synthesize graphene, each with advantages and limitations:
1. Mechanical Exfoliation (Scotch Tape Method)
-
First method used to isolate graphene.
-
Produces high-quality samples, but low yield.
-
Not scalable for industrial use.
2. Chemical Vapor Deposition (CVD)
-
Grows graphene on metal substrates like copper or nickel.
-
Good for large-area, high-quality graphene sheets.
-
Requires high temperature and controlled environment.
3. Reduction of Graphene Oxide (rGO)
-
Involves oxidizing graphite, then reducing it.
-
Cheaper and scalable, but often lower quality than CVD.
Other methods include liquid-phase exfoliation, electrochemical exfoliation, and plasma-enhanced synthesis.
Advantages of Graphene
-
🧠 Ultra-thin and Lightweight
-
⚡ Excellent Electrical & Thermal Conductivity
-
💪 Exceptional Strength-to-Weight Ratio
-
🌫️ Transparent and Flexible
-
🌍 Eco-Friendly Applications in Energy and Water Filtration
These properties position graphene as a superior material for next-generation technologies.
Challenges & Limitations
Despite its vast potential, graphene faces several hurdles:
-
High production costs
-
Difficulty in maintaining consistency across large-scale synthesis
-
Limited commercial applications due to scalability issues
-
Regulatory and environmental concerns around certain production methods
Research is ongoing to overcome these barriers and enable broader industrial adoption.
Applications of Graphene
Graphene is being tested or applied in multiple industries:
✅ Electronics & Semiconductors
-
Flexible displays, transistors, touch screens
-
Ultra-fast processors and memory chips
✅ Energy Storage
-
Graphene batteries (Li-ion, solid-state)
-
Supercapacitors with high charge/discharge cycles
✅ Composites & Coatings
-
Lightweight aerospace components
-
Conductive paints and anti-corrosion coatings
✅ Biomedical
-
Biosensors, drug delivery systems
-
Antibacterial coatings, bioimaging agents
✅ Environmental Solutions
-
Water desalination and filtration membranes
-
Air purification and anti-smog coatings
FAQ: Common Questions About Graphene
Q1: Is graphene the same as graphite?
No. Graphene is a single layer of carbon atoms, while graphite is a stack of many graphene layers.
Q2: Can graphene replace silicon in chips?
Graphene has superior conductivity, but challenges in creating a bandgap limit its use as a silicon replacement—for now.
Q3: Is graphene available in consumer products?
Yes, graphene is now found in sports gear, clothing, phone batteries, and even face masks—but widespread adoption is still developing.
Q4: Is graphene toxic?
Most studies show graphene is biocompatible, but research continues on its long-term environmental and health effects.
The Future of Graphene
The global graphene market is projected to grow at over 30% CAGR through 2030. With continual advances in low-cost production and integration into hybrid materials (e.g., graphene + CNTs), the future of graphene includes:
-
Next-gen wearables and implantable electronics
-
High-capacity graphene batteries for EVs and smart grids
-
Intelligent filtration systems for clean water and air
-
Smart composites for aerospace, automotive, and construction
As industrial scaling becomes viable, graphene will play a vital role in building a more sustainable, efficient, and connected world.
Conclusion
Graphene is not just a scientific marvel—it’s a material with transformative real-world applications. From electronics to energy, its unique properties can redefine how we design and use technology. While challenges remain, the momentum behind graphene research and commercialization continues to accelerate.
📩 Want to stay ahead in nanomaterials innovation?
Subscribe to our newsletter or contact us for the latest in graphene development and partnership opportunities.

