Efficient One-Second Flash Synthesis of Graphene from Carbon Sources
Introduction
Graphene synthesis on a large scale primarily uses bottom-up methods by mechanically exfoliating carbon atoms from graphite layers. These methods often require significant solvent and energy inputs, such as mixing, shearing, ultrasound, or electrochemical processing. Chemical oxidation to convert graphite to graphene oxide aids exfoliation but introduces defects during reduction. Conversely, high-quality graphene synthesis via chemical vapor deposition or advanced organic synthesis yields small quantities, while solution-based methods often produce defective products.
Flash Joule Heating Method
Our study demonstrates that using flash Joule heating, inexpensive carbon sources like coal, petroleum coke, biochar, carbon black, waste food, rubber tires, and mixed plastic waste can synthesize gram-scale graphene in one second. This product, termed “Flash Graphene” (FG), exhibits disordered but random layer stacking. This method does not require furnaces, solvents, or reactive gases. The yield depends on the carbon content of the raw materials; high-carbon sources like carbon black, anthracite, or sintered coke achieve yields of 80-90% with purity over 99%, needing no further refinement. Raman spectroscopy shows FG with weak or absent D peaks, indicating it may be one of the least defective graphenes. The disordered interlayer structure facilitates rapid layering in composites. The energy cost per unit product is only 7.2 kJ/g, making it promising for large-scale composite applications in plastics, metals, wood, and concrete.
Graphene Synthesis Analysis
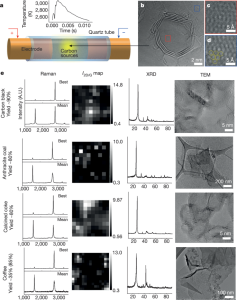
- Figure 1: Shows TEM images of FG synthesized from carbon black (CB-FG), indicating Moiré patterns due to disordered layer stacking. Raman spectra, XRD patterns, and TEM images of FG from various raw materials are also provided. Low D peak intensity in Raman spectra from carbon black and petroleum coke indicates low defect density. XRD results show larger FG flake size from coffee grounds, and TEM reveals micron-sized folded structures from coffee grounds and coal.
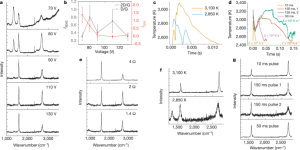
- Figure 2: Highlights the importance of high temperature (above 3000K) for low-defect FG synthesis. Different voltage and compression times affect temperature-time curves, indicating that a brief flash (<10ms) yields higher I2D/IG ratios. Compression enhances conductivity and shortens discharge time.

- Figure 3: Molecular dynamics simulations show rapid FG growth at the molecular level, with high temperatures (5000K) favoring quick FG formation. Simulations align with experimental results, elucidating the mechanisms of FG synthesis.
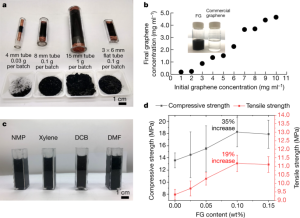
- Figure 4: Compares FG synthesis using different quartz tube sizes, showing higher yields and quality with flat tubes. FG disperses well in water and organic solvents due to its disordered interlayer structure. In cement composites, 0.1% CB-FG significantly improves compressive and tensile strengths.
Conclusion
This novel low-cost bottom-up synthesis method efficiently converts low-cost carbon sources (like coal and petroleum coke), renewable resources (like biochar and rubber tires), and mixed wastes (including plastic bottles and food waste) into easily dispersible turbostratic graphene (Flash Graphene, FG) using high-voltage electric current to rapidly heat the carbon source above 3000K in one second. Optimizing key parameters like reaction temperature and time achieves high-quality FG with high I2D/IG ratios and low defect density. FG’s disordered interlayer structure enhances dispersion in composites, significantly improving performance in applications like cement. Molecular dynamics simulations support these findings, providing insights into FG’s rapid molecular growth mechanisms. Scaling up FG synthesis demonstrates its potential for large-scale industrial applications, especially in building materials.
Source: Nature Journal Article

