Lithium-ion batteries (LIBs) have become indispensable in powering modern devices, from electric vehicles (EVs) to consumer electronics. However, safety concerns—particularly thermal runaway, a chain reaction where increasing temperature leads to catastrophic failure—pose significant risks. Addressing this challenge is paramount to ensuring widespread adoption of LIBs. Carbon materials, with their unique thermal, mechanical, and electrical properties, have emerged as critical components in mitigating thermal runaway risks. This article explores the causes of thermal runaway, examines the limitations of traditional safety measures, and highlights how carbon materials like graphene, carbon nanotubes (CNTs), and carbon-coated separators provide innovative safety solutions.
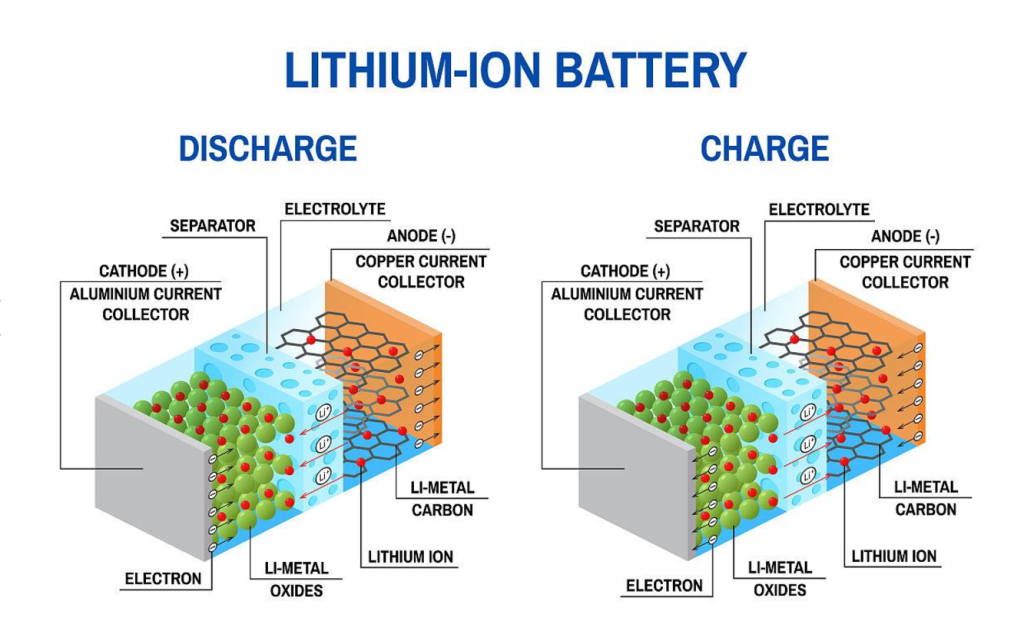
Understanding Thermal Runaway in Lithium-Ion Batteries
1. What is Thermal Runaway?
Thermal runaway occurs when a LIB experiences uncontrolled heat generation due to internal or external factors. This reaction typically follows these stages:
- Overheating: Triggered by overcharging, high-current discharge, or external heat.
- Electrolyte Decomposition: Releases flammable gases, further increasing pressure and heat.
- Electrode Reaction: Exothermic reactions between the electrolyte and electrodes exacerbate temperature spikes.
- Fire or Explosion: If unchecked, thermal runaway can lead to battery failure, fires, or even explosions.
2. Common Causes
- Short Circuits: Internal or external short circuits cause local overheating.
- Mechanical Damage: Physical stress or punctures can lead to separator failure.
- Overcharging: Excessive voltage destabilizes the electrolyte and electrodes.
- Poor Heat Dissipation: Inadequate thermal management amplifies overheating.
Traditional Safety Measures and Their Limitations
Existing safety measures, including thermal management systems, protective circuits, and electrolyte additives, have limitations:
- Complexity: Thermal management systems add weight and cost, making them less practical for large-scale EV applications.
- Inefficiency: Conventional separators are limited in thermal stability, and protective circuits cannot always prevent internal failures.
- Limited Scalability: Many advanced safety technologies remain cost-prohibitive for mass production.
Carbon Materials: Advanced Solutions for Battery Safety
1. Graphene: A Heat-Dissipating Shield
Graphene, a two-dimensional carbon material, offers exceptional thermal conductivity (up to 5300 W/m·K) and mechanical strength. By enhancing heat dissipation, graphene prevents localized overheating.
Example:
In a study by Tsinghua University, graphene-coated electrodes reduced hot-spot formation by 40%, significantly lowering the risk of thermal runaway. Graphene’s ability to rapidly transfer heat away from critical areas ensures stable battery operation even under high current loads.
2. Carbon Nanotubes (CNTs): Building Resilient Conductive Networks
CNTs improve both electrical and thermal conductivity. When integrated into electrodes, CNTs create a conductive network that reduces resistance and evenly distributes heat.
Example:
Samsung researchers incorporated CNTs into anode materials, achieving a 25% reduction in resistance and a corresponding drop in heat generation during high-rate charging. This breakthrough minimizes thermal stress, enhancing overall battery safety.
3. Carbon-Coated Separators: Enhancing Thermal Stability
Battery separators play a critical role in preventing short circuits by keeping the anode and cathode apart. Carbon-coated separators offer superior thermal and mechanical stability compared to traditional polymer separators.
Example:
Japanese company Toray Industries developed carbon-coated separators capable of withstanding temperatures up to 200°C without melting. This prevents separator shrinkage—a common cause of thermal runaway—under high-temperature conditions.
4. Carbon Black: Preventing Overheating at Minimal Cost
Carbon black, an affordable conductive additive, enhances the uniformity of electrode materials, preventing localized overheating.
Example:
A study by CATL demonstrated that adding carbon black to cathode slurries reduced thermal gradients during operation, cutting the likelihood of hot-spot formation by 15%. Its low cost makes it suitable for large-scale applications, such as EV batteries.
Carbon Materials in Thermal Management Systems
1. Graphene-Based Thermal Pads
Graphene-based thermal interface materials (TIMs) improve heat dissipation between battery cells and cooling systems.
Example:
A partnership between Tesla and a graphene manufacturer resulted in the integration of graphene TIMs in EV battery packs. These materials reduced operational temperatures by 10°C, extending battery lifespan and preventing thermal runaway under extreme conditions.
2. Carbon Foams for Heat Insulation
Carbon foams, with their porous structure and low thermal conductivity, act as effective heat barriers, isolating hot spots.
Example:
BMW uses carbon foam in its battery modules to insulate cells and delay thermal propagation in the event of a failure. This additional layer of protection provides precious time for cooling systems to respond.
Innovations in Carbon-Based Electrolyte Additives
Carbon materials can also enhance the thermal stability of electrolytes. For example, graphene oxide (GO) additives increase the decomposition temperature of electrolytes, reducing the likelihood of thermal runaway.
Example:
In a collaboration between the Chinese Academy of Sciences and industrial partners, GO-enhanced electrolytes raised thermal decomposition thresholds by 20°C, enabling safer operation in high-temperature environments.
Reducing Thermal Propagation with Carbon Materials
One of the greatest risks of thermal runaway is thermal propagation—where the failure of one cell triggers neighboring cells. Carbon materials offer solutions by:
- Improving Thermal Barriers: Graphene coatings on cell casings prevent heat transfer between cells.
- Absorbing Heat: Carbon nanotube sheets within battery modules absorb excess heat, mitigating propagation risks.
Example:
LG Chem implemented CNT-based heat shields in its battery packs, achieving a 30% reduction in thermal propagation rates during safety tests.
Environmental and Cost Advantages of Carbon Materials
Beyond enhancing safety, carbon materials contribute to environmental and cost benefits:
- Sustainability: Biomass-derived graphene and recycled carbon black offer eco-friendly alternatives to traditional materials.
- Cost-Effectiveness: Integrating carbon materials reduces the need for complex thermal management systems, cutting production costs by 10–15%.
Example:
An EV manufacturer in China reported saving $200 per battery pack by replacing conventional thermal management components with graphene-enhanced solutions.
Future Outlook: Scaling Carbon Material Integration
The scalability of carbon materials remains a challenge but is rapidly improving:
- Mass Production: Advances in chemical vapor deposition (CVD) and other scalable methods are lowering production costs for graphene and CNTs.
- Hybrid Solutions: Combining multiple carbon materials (e.g., graphene and CNTs) offers synergistic safety benefits.
Conclusion
Thermal runaway remains a critical challenge in lithium-ion battery safety. Carbon materials, including graphene, carbon nanotubes, carbon-coated separators, and carbon black, provide innovative solutions to mitigate these risks. Through enhanced thermal conductivity, improved mechanical stability, and effective heat dissipation, these materials significantly reduce the likelihood of thermal runaway while offering cost and environmental benefits. As research and industrial adoption progress, carbon materials are set to play a vital role in shaping the future of safer, more efficient energy storage technologies.

