Graphene Oxide Advances T-Cell-Based Immunotherapy in Cancer Treatment – New Publication in Nature Nanotechnology
Introduction
T-cell-based immunotherapies, especially CAR-T cell therapies, have transformed cancer treatment since the FDA’s approval in 2017. The effectiveness of CAR-T therapies depends on the efficient in vitro activation and expansion of functional T-cells and the transfer of engineered CAR genes. Current T-cell activation methods, using anti-CD3 and anti-CD28 antibodies on rigid surfaces (e.g., microbeads or plates), simulate natural immune activation but still require external IL-2 supplementation, unlike the autonomous IL-2 production seen in natural immune synapses.
In response to this gap, researchers from UCLA, including Yu Huang and Lili Yang, developed a flexible, graphene oxide-based antigen-presenting platform (GO-APP). The GO-APP mimics in vivo immune synapses more effectively than conventional rigid platforms, offering improved T-cell activation, expansion, and CAR transduction efficiency without the need for external IL-2.
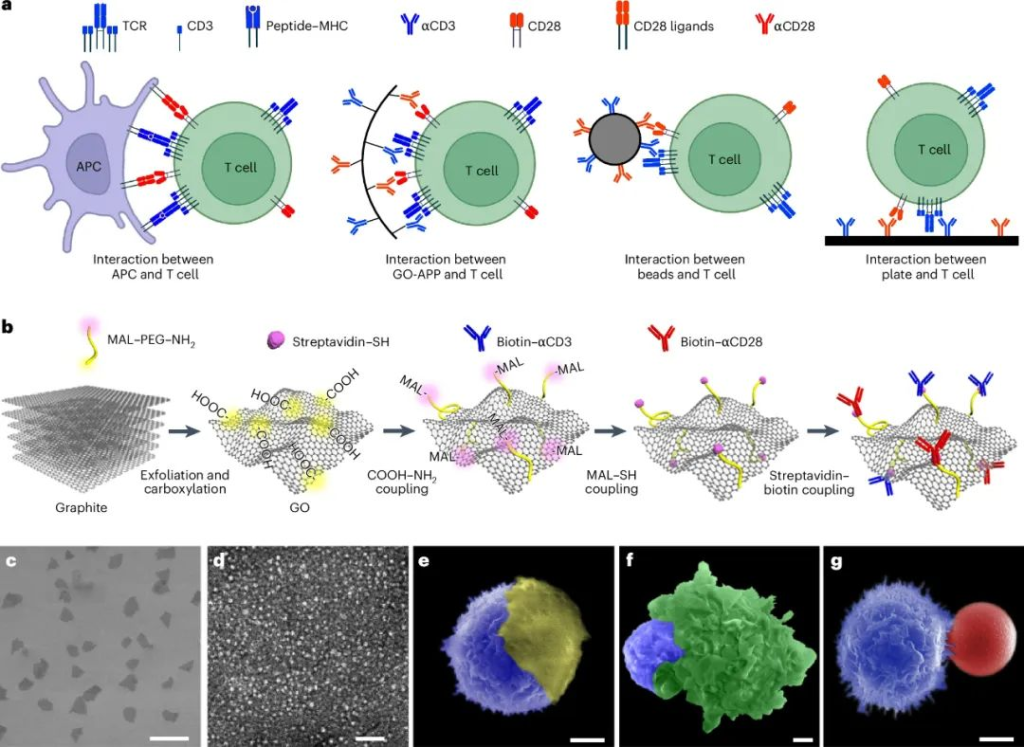
Design and Characterization of the Graphene Oxide Platform (GO-APP3/28)
The researchers synthesized graphene oxide (GO) sheets using an improved Hummers method, with an average size of 11.8 ± 2.6 μm and thickness of 1.0 ± 0.2 nm. GO surfaces were modified by introducing carboxyl groups through chloroacetic acid treatment and further conjugated with anti-CD3 (αCD3) and anti-CD28 (αCD28) antibodies using flexible polyethylene glycol (PEG) linkers. Each GO sheet contained approximately 1119 antibody molecules per μm², maximizing T-cell interaction.
- GO-APP3/28 Contact Area: The platform achieved a T-cell contact area of 75.82 ± 2.59 μm², comparable to the natural immune synapse. This is significantly larger than that of commercial Beads3/28, providing better surface flexibility and interaction.
- Optimal Concentration: GO-APP3/28 showed the best T-cell activation at 0.1 μg/mL, aligning with commercial microbeads’ optimal concentration.
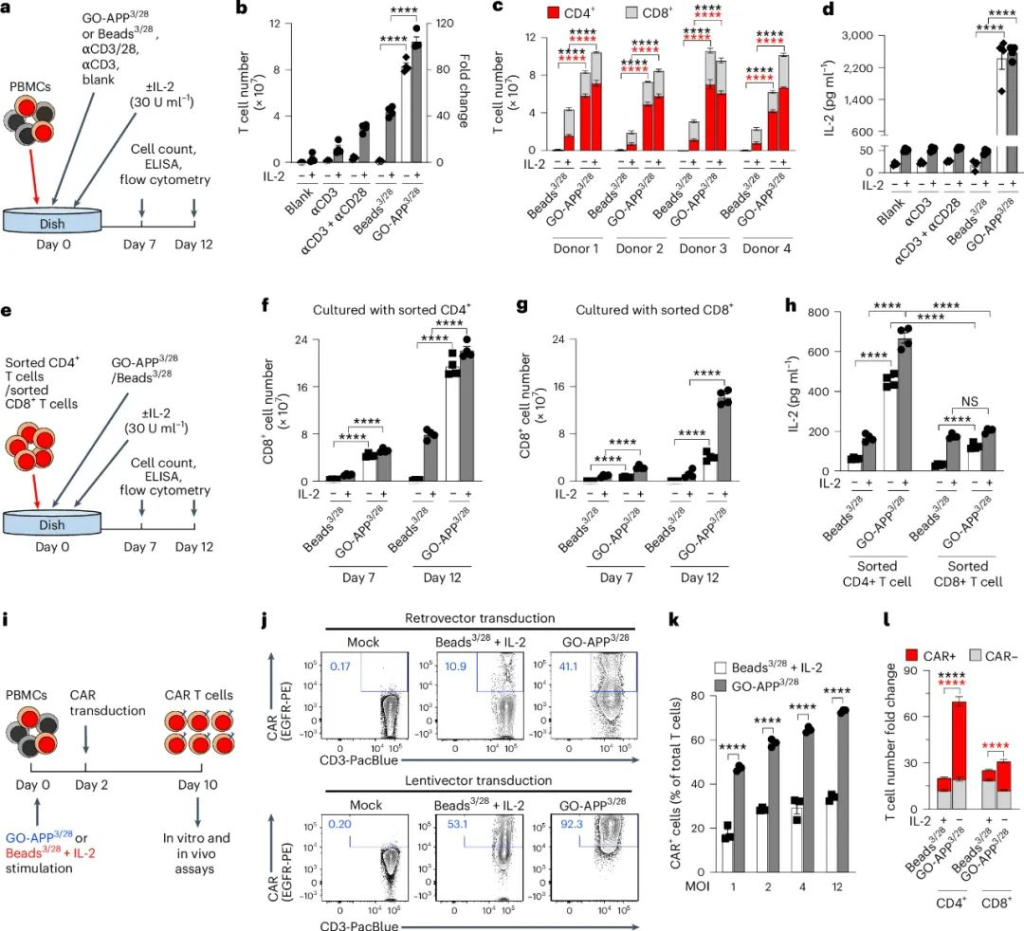
GO-APP3/28 in T-Cell Expansion and CAR-T Cell Production
GO-APP3/28 outperformed traditional Beads3/28 in activating and expanding T-cells, even without IL-2 supplementation. Key findings include:
- Higher Cytokine Production: Activated T-cells produced more IL-2 and IFNγ, enhancing their survival and proliferation.
- Improved CAR-T Transduction Efficiency: GO-APP3/28 enabled more efficient CAR gene transfer with reduced viral vector usage, lowering production costs.
- Enhanced CD4+ and CD8+ T-Cell Expansion: The platform improved the separation and activation of both CD4+ and CD8+ T-cell subsets.
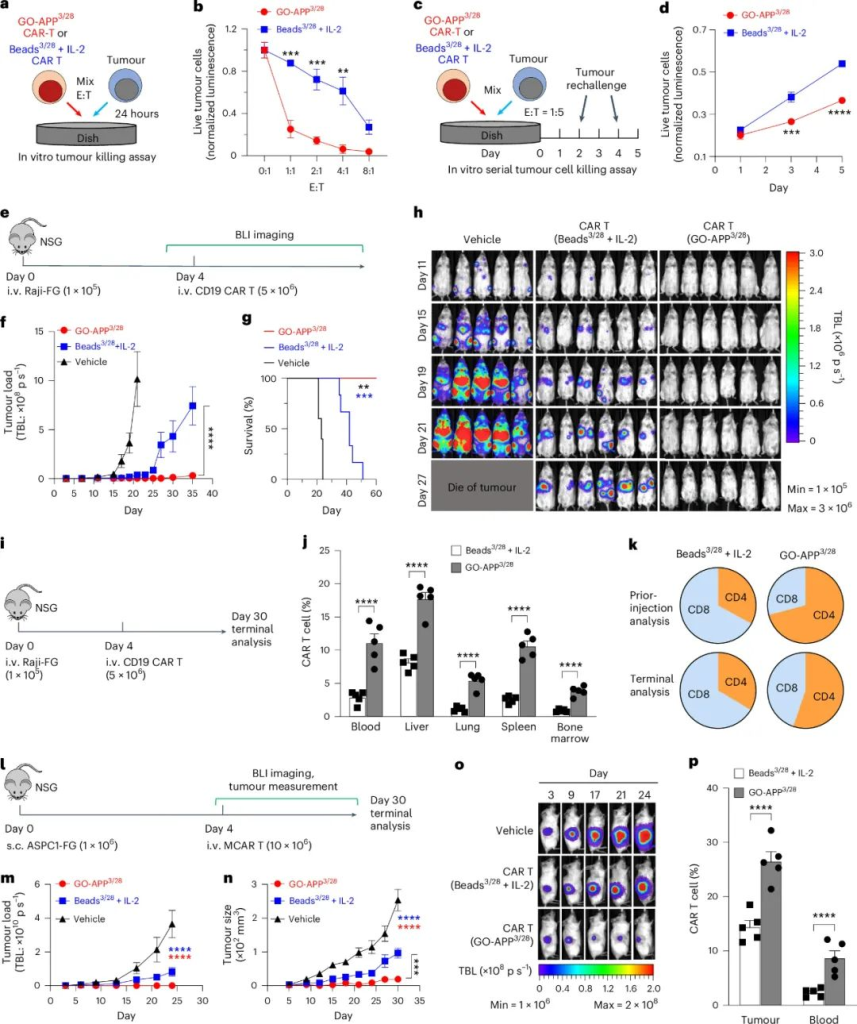
GO-APP3/28 in CAR-T Cell Cancer Therapy
In both in vitro and in vivo tumor models, GO-APP3/28-activated CAR-T cells showed superior antitumor effects compared to microbead-activated CAR-T cells.
- Tumor Killing: GO-APP3/28 CAR-T cells demonstrated stronger initial and sustained tumor-killing abilities in continuous tumor challenge assays.
- In Vivo Efficacy: In mouse models of Raji lymphoma and AsPC-1 pancreatic cancer, GO-APP3/28 CAR-T cells provided:
- Longer survival times and lower tumor burdens.
- Better persistence of T-cells in multiple tissues, enhancing therapeutic outcomes.
Mechanism of T-Cell Activation with GO-APP3/28
Single-cell RNA sequencing revealed distinct gene expression profiles in T-cells activated by GO-APP3/28 versus Beads3/28. GO-APP3/28-activated cells showed:
- Upregulation of key T-cell activation pathways: CD3, CD28, IL-2, and TCR signaling pathways were more active.
- Enhanced Effector Functionality: CD8+ T-cells exhibited multifunctional effector phenotypes, while CD4+ T-cells produced higher levels of IL-2.
- Selective Polarization: GO-APP3/28 promoted TH1 and TH2 responses without polarizing towards TH17 or Treg phenotypes.
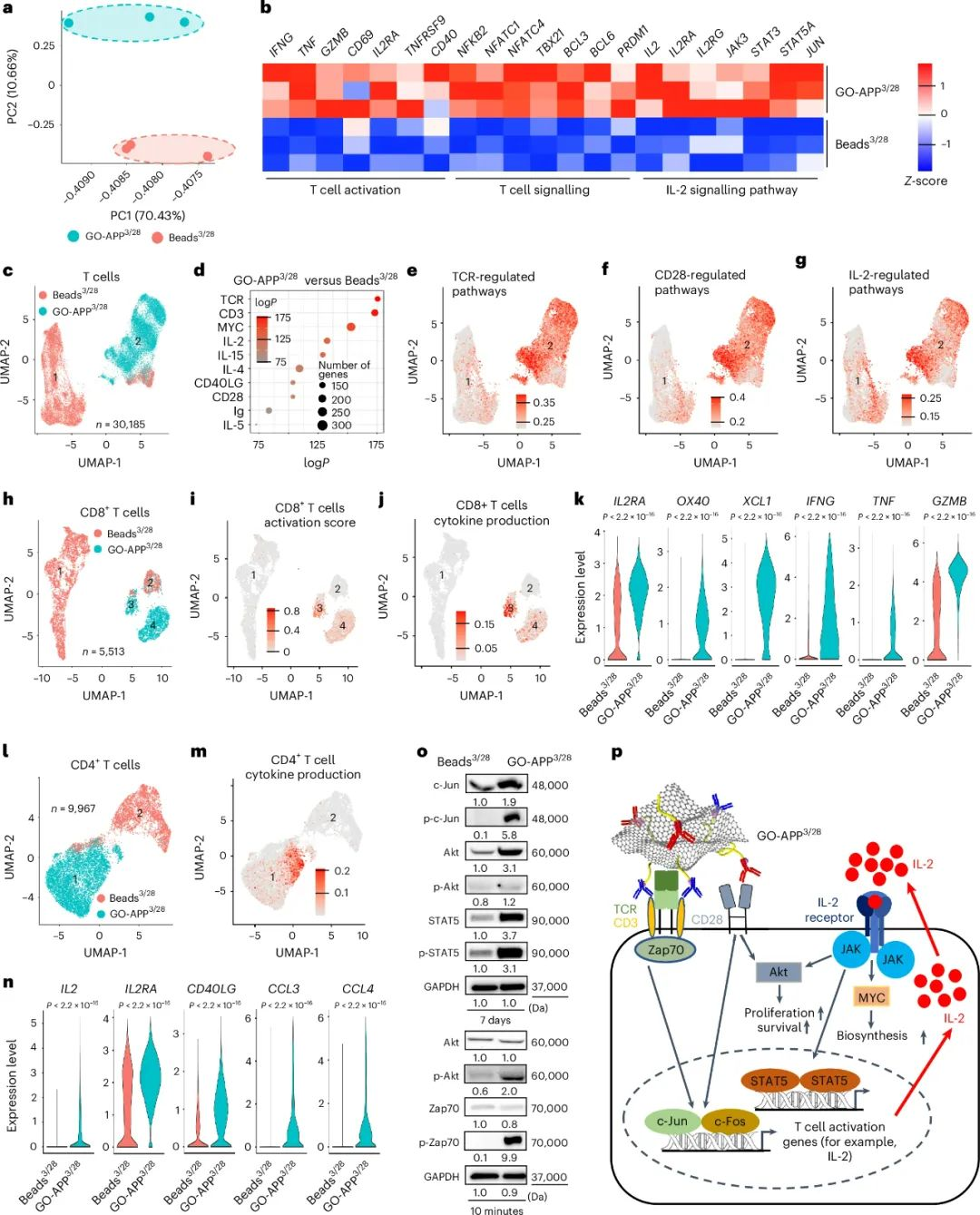
Increased protein expression and phosphorylation in key pathways confirmed that GO-APP3/28 more closely mimics natural immune activation than rigid platforms.
Conclusion and Future Directions
GO-APP3/28 represents a significant advancement in T-cell activation platforms by offering a flexible, biomimetic structure that closely mimics the immune synapse. It enables high-capacity T-cell expansion while maintaining robust effector functions and improves CAR-T cell manufacturing efficiency by reducing reliance on viral vectors and external IL-2.
- Biocompatibility: GO-APP3/28 exhibited no toxicity in vitro or in vivo, mitigating concerns about GO materials.
- Separation Challenges: Future work may focus on integrating magnetic nanoparticles into GO-APP3/28 to facilitate easier separation from cell products.
GO-APP provides a new strategy for exploring cell signaling interactions and developing therapeutic applications. Its flexible design opens new avenues for immunological research and T-cell-based therapies.
Reference:
Zhu, E., Yu, J., Li, Y.R., et al. Biomimetic cell stimulation with a graphene oxide antigen-presenting platform for developing T cell-based therapies. Nat. Nanotechnol. (2024).
https://doi.org/10.1038/s41565-024-01781-4

