Next-Generation Battery Material Solution: High-Performance Anode Binders
With the rapid growth of the new energy and storage markets, high-performance batteries are increasingly required to meet diverse application needs. The demand for batteries with high safety, high energy density, and long cycle life continues to rise. In response, LG Chem has been intensifying its research and development efforts in battery materials, especially focusing on anode binders to enhance lithium-ion battery performance further.
Role of Anode Binders

Anode binders play a crucial role in the chemistry of batteries by adhering the “active material” that participates in electrochemical reactions with the “conductive materials” that enable electron movement to the current collector film. By mixing and uniformly applying these components with binders, batteries can achieve stable performance and strong adhesion at the electrode interface, as well as inside the battery. This bonding is essential for the mechanical durability of the electrode structure.
- Current Collector: Collects and outputs the current generated by the active material while also supplying current to the active material for electrochemical reactions during battery charge and discharge.
Types and Mechanism of Anode Binders
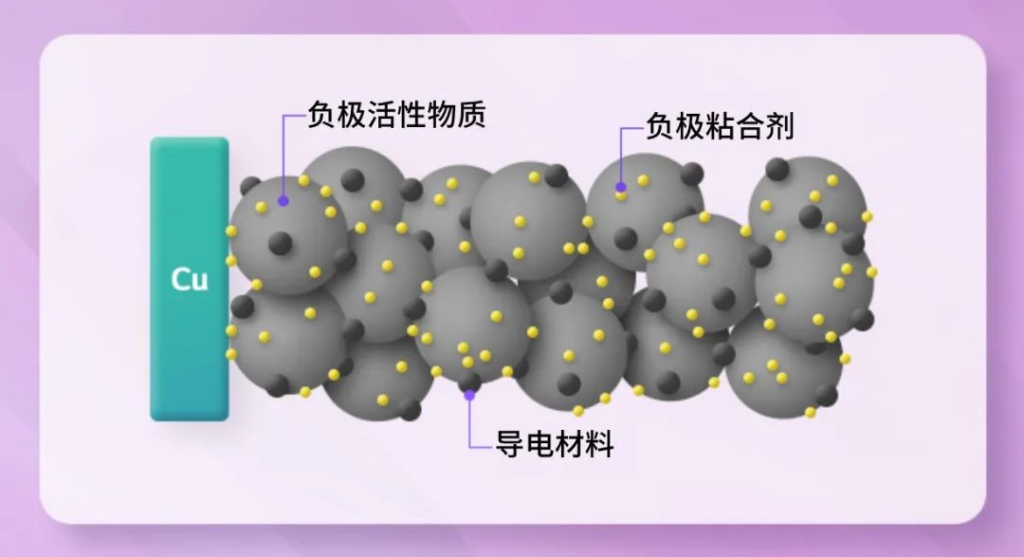
Binders, as non-conductive insulators, can be used in both cathode and anode materials. They are generally divided into the following types based on application:
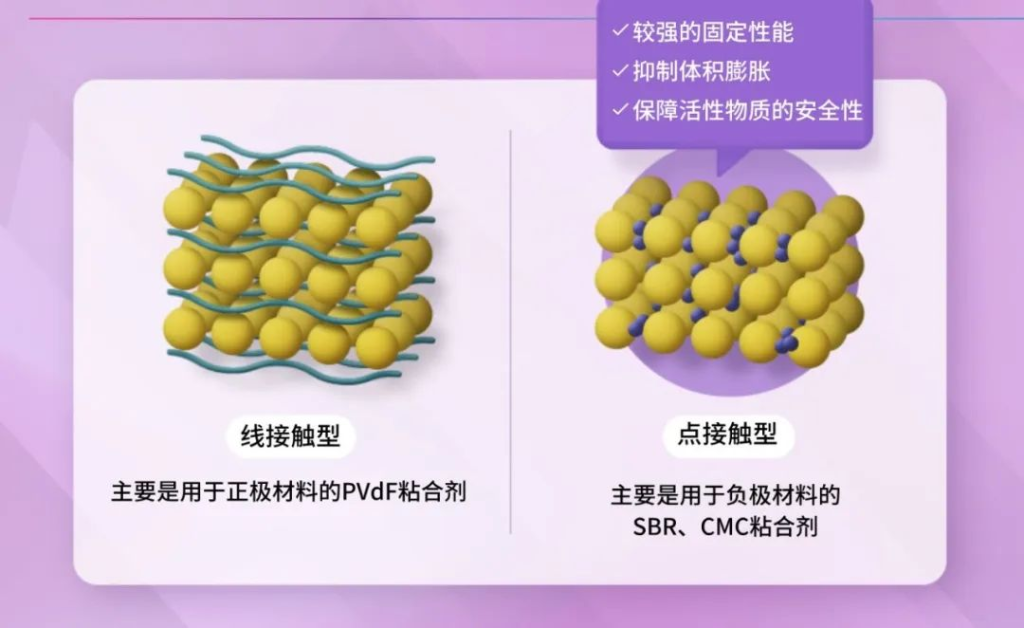
- Line-Contact Binders (PVdF): Polyvinylidene fluoride (PVdF) is a non-aqueous binder, which does not dissolve in water and incurs a certain recycling cost.
- Point-Contact Binders (SBR & CMC): Styrene-butadiene rubber (SBR) and carboxymethyl cellulose (CMC) are water-based binders that are environmentally friendly and cost-effective. These binders provide better stability than PVdF.
Cathode materials typically use low-expansion metals such as nickel, cobalt, and manganese, and therefore, non-aqueous PVdF binders are usually sufficient. However, the anode often utilizes materials with significant volume changes during charge and discharge, necessitating the use of water-based SBR and CMC binders.
LG Chem’s Anode Binder Solution
As the demand for increased battery capacity grows, more silicon is being added to anode materials, with silicon usage now reaching up to 10 times that of traditional graphite. However, silicon expands significantly during battery charge and discharge cycles, leading to battery degradation. Poor binder adhesion exacerbates this issue, increasing the gap between active materials, reducing electron migration, and slowing down electron transport.

To address this challenge, LG Chem has incorporated carbon nanotubes (CNTs) as conductive materials and developed high-performance binders specifically designed for these requirements. Through functional monomers and optimized binder particle composition and structure, LG Chem minimizes coagulation between binders, enhancing their bonding points with active materials. This design improves adhesion efficiency and boosts the bond strength between conductive materials and active substances. Furthermore, it reduces binder migration to the electrode surface during drying, ensuring uniform distribution within the electrode and improved adherence to active materials and current collectors.
LG Chem’s anode binders offer excellent electrochemical stability and cycling characteristics, maintaining mechanical stability for the anode active material and significantly reducing internal resistance increase. These binders are primarily used in lithium-ion batteries for electric vehicles and energy storage systems (ESS).

Source: LG Chem

