Osaka University ACS ML: Covalently-Converted Graphene Mediated Homogeneous Organohydrogel for Multimodal Perception and Soft Actuation Electronic Skin
Source: Materials Analysis and Applications, Author: Carbon_Art
Materials Analysis and Applications shares news and insights from the field of materials science.
Summary of Results
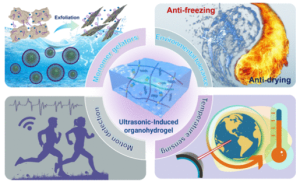
Bioelectronic devices based on common hydrogels containing conductive components face significant issues such as poor structural compatibility, compromised signal accuracy, and fatigue failure in harsh environments, which limit their multifunctionality. To address the problems of additive agglomeration and phase separation in polymer matrices, Osaka University researchers Hiroshi Uyama et al. published an innovative method in the journal ACS Materials Letters. The study, titled “Homogenous Organohydrogel Mediated by Covalently-Converted Graphene Nanosheets as an Electronic Epidermis for Multimodal Perception and Soft Actuation,” proposes the assembly of amphiphilic nanosheets at the oil/water interface to achieve cost stability.
With the aid of ionic liquid (IL) grafting-peeling, highly dispersed graphene nanosheets can be gelled through ultrasonication and chemically integrated into a swelling-resistant polymer network. Furthermore, the synergistic effect between the dimethyl sulfoxide (DMSO)/H2O binary solvent and charged polar terminal groups weakens hydrogen bonds within water molecules, endowing the organohydrogel with reliable environmental tolerance and long-lasting moisture retention. The rapidly prepared organohydrogel boasts high mechanical stretchability, satisfactory sensitivity, and excellent photothermal conversion properties, making it suitable for all-weather wearable sensors for daily activity monitoring and temperature sensing, thus laying the foundation for human-machine interaction and thermally induced actuation.
Detailed Analysis
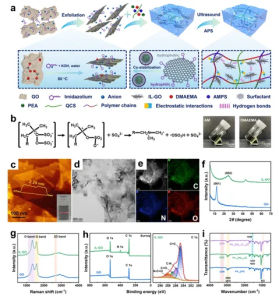
Figure 1 (a) Schematic of the preparation process and interactions of IL-GO and PAxDGyPz/Q organohydrogel. (b) Illustration of radical generation and images of rapidly polymerized organohydrogel. (c, d, e) Atomic force microscopy (AFM) and transmission electron microscopy (TEM) images of IL-GO, and corresponding energy-dispersive X-ray spectroscopy (EDS) element maps. (f, g) X-ray diffraction (XRD) and Raman spectra comparisons between original GO and IL-GO. (h) X-ray photoelectron spectroscopy (XPS) survey spectra of GO and IL-GO, with high-resolution C 1s spectra inset. (i) ATR-FTIR spectra of PA1.5D/Q, PA1.5DG0.75/Q, and PA1.5DG0.75P1.25/Q organohydrogels.
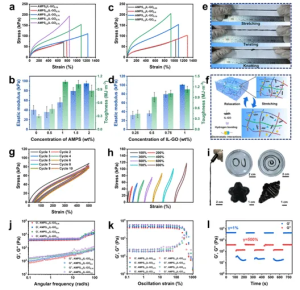
Figure 2 (a, b) Typical strain-stress curves of organohydrogels with varying AMPS content, and calculations of corresponding elastic modulus and toughness. (c, d) Strain-stress curves and Young’s modulus, and toughness of organohydrogels with different IL-GO contents. (e) Photos illustrating high deformation, including continuous stretching, twisting, and knotting. (f) Diagram of internal structure diversification during stretching and relaxation. (g, h) Continuous loading-unloading stress-strain curves at 500% fixed strain, 10 cycles, with strain increasing from 100% to 800%. (i) Pinhole extrusion and shape adaptability of organohydrogel fibers. (j, k) Angular frequency scans and dynamic shear scan measurements of organohydrogels with different IL-GO contents. (l) Cyclic step strain measurements of PA1.5DG0.75P1.25/Q organohydrogel between 1% and 500% strain.
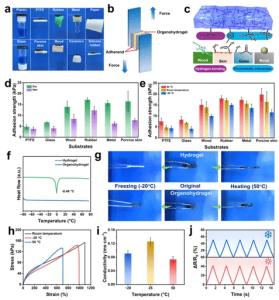
Figure 3 (a) Photos showing adhesion properties of PA1.5DG0.75P1.25/Q organohydrogel with various substrates (plastic, PTFE, rubber, metal, paper, glass, pigskin, wood, ceramic, and silicone rubber). (b, c) Lap shear test and adhesion mechanism diagrams. (d, e) Adhesion strength of PA1.5DG0.75P1.25/Q organohydrogel to different substrates under dry, wet, room temperature, 50°C, and -20°C conditions. (f, g) Heat flow curves and photos showing hydrogels and organohydrogels after 24-hour extreme condition treatment. (h-j) Tensile strength, conductivity, and relative resistance change of organohydrogel strain sensors at -20°C and 50°C up to 50% strain.
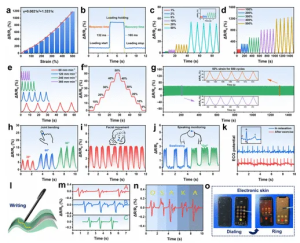
Figure 4 (a) Relative resistance changes with strain range 0-500%. (b) Response and recovery times during loading-unloading tests. (c, d) Sensitivity under small (1-50%) and large (100-500%) strains. (e) Sensitivity at representative elongation rates. (f) Relative resistance change under stepwise strain. (g) Durability test reflecting relative resistance changes at 50% strain for 500 continuous cycles. (h-j) Relative resistance recordings during finger bending, facial movements, swallowing, and speaking. (k) ECG signal collected by the organohydrogel electrode. (l) Writing board schematic. (m, n) Resistance changes in the handwriting board when writing representative letters A, B, and C three times consecutively. (o) Photos showing the organohydrogel as electronic skin during dialing.
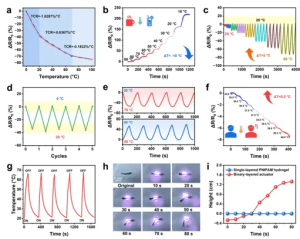
Figure 5 (a) Relative resistance changes with temperature. (b) Relative resistance changes when temperature drops from 90°C to 10°C. (c) Time-relative resistance changes during cyclic heating and cooling processes. (d) Temperature responsiveness over five cycles from 0°C to 20°C. (e) Real-time relative resistance changes during continuous heating-cooling and cooling-heating cycles. (f) Real-time relative resistance changes simulating body temperature increase from 35°C to 40°C with a temperature gradient of 0.5°C. (g) Temperature change curves of PA1.5DG0.75P1.25/Q organohydrogel over five near-infrared light on/off cycles. (h) Optical images showing the actuator’s driving behavior under near-infrared irradiation. (i) Actuator curves reflecting high temporal changes.
Conclusion
In summary, we designed an alkylated IL that achieves polydisperse covalent conversion of graphene through nucleophilic ring-opening reactions and innovatively expands its function as a two-dimensional surfactant. The polymerizable IL-GO is integrated with the ultrasonically induced organohydrogel network, rather than merely blending. The rapidly prepared organohydrogel exhibits good mechanical performance (strength of 160 kPa, elongation at break of 1090%), amphibious adhesion, moisture retention, environmental tolerance (-20 to 50°C), and conductivity, making it suitable as a multimodal all-weather sensor for various scenarios. The electronic skin (GF = 3.43 at 300-500% strain) demonstrates reliable sensing capabilities for real-time monitoring of body movements, physiological signal recording, handwriting recognition, and use as a touchscreen stylus. Its excellent temperature sensing capability (TCR = -1.8287%/°C, -0.6367%/°C, and -0.1832%/°C) allows for instantaneous temperature change detection, and its superior photothermal conversion efficiency by IL-GO shows great potential for near-infrared actuation (90° in 90 seconds). This work provides new perspectives for developing multifunctional soft materials, inspiring the future design and manufacturing of multifunctional electronic skins and near-infrared light-induced actuators.
Reference:
https://doi.org/10.1021/acsmaterialslett.4c00809
Source: Materials Analysis and Applications

