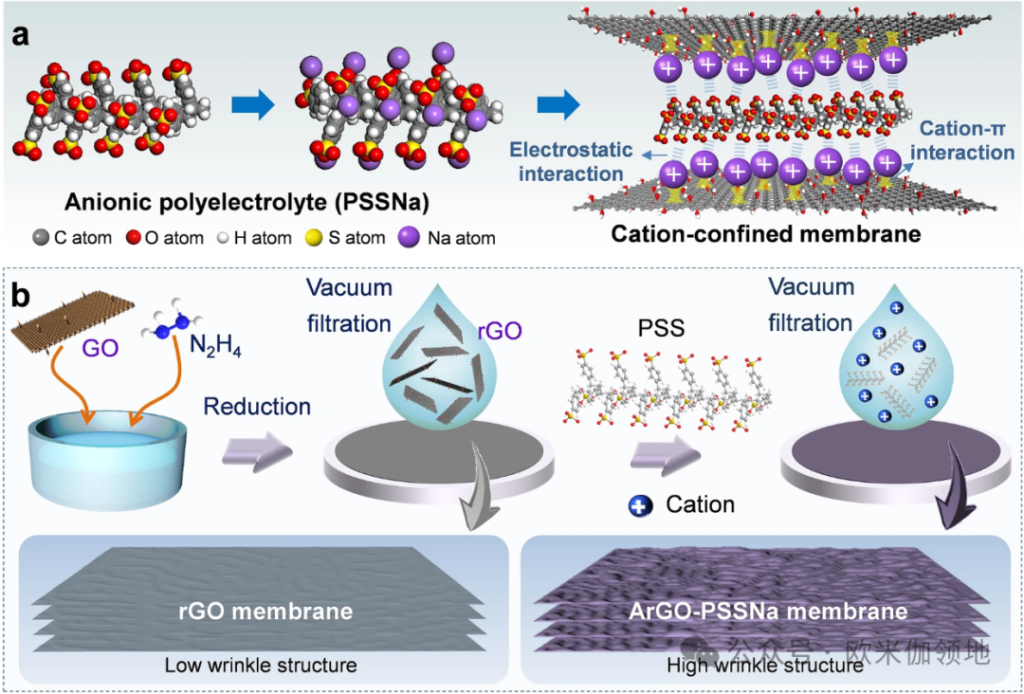
Figure 2: Preparation of the ArGO-PSSNa Membrane
Researchers first assembled PSSNa onto amine-crosslinked rGO nanosheets to create the cation-restrictive ArGO-PSSNa membrane (Figure 2b). PSSNa, rich in negatively charged sulfonate groups, attracts cations from water into the membrane’s nanochannels. The rGO nanosheets interact with cations through cation–oxygen and cation–π electron interactions, allowing the membrane to enrich and restrict cations within the nanochannels (Figure 2a). SEM images show the rGO membrane as smooth with slight wrinkles, with a thickness of around 37 nm, while the ArGO-PSSNa membrane shows pronounced wrinkles and is about 63 nm thick. TEM images indicate that the rGO membrane has a layered, smooth, and homogeneous structure with an interlayer spacing of 1 nm; in contrast, the ArGO-PSSNa membrane has numerous 1 nm-sized spots, with interlayer spacing increased to 1.3 nm, suggesting PSSNa incorporation into the rGO layers.
The XRD spectrum of the GO membrane reveals a characteristic peak at 9.4°, which shifts to 9.1° (interlayer spacing of 9.7 Å) in the rGO membrane. After amine crosslinking, the ArGO membrane’s peak shifts further to 8.6°, and the ArGO-PSSNa membrane’s peak shifts to 6.9° (spacing of 12.8 Å). 2D wide-angle XRD (2D-WAXD) patterns show that GO’s diffraction arc transforms into rGO’s diffraction spot, indicating that rGO layers stack more regularly. In contrast, amine crosslinking and PSSNa insertion lead to disordered stacking in the ArGO-PSSNa membrane, as indicated by the transformation of the diffraction pattern back into an arc.
EDS mapping and XPS analysis confirm that the ArGO-PSSNa membrane contains C, O, N, S, and Na. XPS spectra indicate a higher C content (96.3 at.%) in rGO membranes compared to GO, while the ArGO-PSSNa membrane has a reduced C content of 83.2 at.% and higher levels of N, S, and Na at 5.3, 4.6, and 3.7 at.%, respectively. FTIR spectra further confirm the presence of C–N, N–H, S–O, and C–S bonds, validating amine crosslinking and PSSNa incorporation in the composite membrane. The zeta potential of ArGO-PSSNa reaches -102.4 mV, significantly higher than that of rGO and ArGO membranes (-20.3 and -46.4 mV), indicating a high degree of negative charge.
The ArGO-PSSNa membrane’s pure water permeability is 48.6 L m-2h-1 bar-1, 4.5 times that of GO (10.7 L m-2h-1 bar-1) and 25.6 times that of rGO (1.9 L m-2h-1 bar-1). The dynamic water contact angle reduction rate for GO is 6.57 °/min, for rGO it is 8.16 °/min, and for ArGO-PSSNa it is 13.3 °/min, indicating enhanced wettability due to PSSNa, which reduces water transport resistance in the nanochannels. The surface free energy of the ArGO-PSSNa membrane is 55.3 mJ m-2, higher than that of the rGO (46.5 mJ m-2) and GO (52.2 mJ m-2) membranes, showing that high surface free energy correlates with improved water permeability.
When filtering a 5 mM NaCl solution, the GO membrane achieves a permeability of 5.5 L m-2h-1 bar-1 and NaCl rejection of 26.5%, while the rGO membrane achieves 1.7 L m-2h-1 bar-1 and 12.8% rejection. The ArGO-PSSNa membrane reaches 23.0 L m-2h-1 bar-1 and 95.5% rejection. Over 40 hours of filtration, the ArGO-PSSNa membrane consistently maintains 19-23 L m-2h-1 bar-1 permeability with 90-95% NaCl rejection. Additionally, the ArGO-PSSNa membrane achieves over 96% rejection for Na2SO4 and MgSO4 and maintains over 90% rejection for KCl and MgCl2, indicating its effective rejection of various salts.
Subsequently, researchers evaluated ion transport through GO, rGO, ArGO, and ArGO-PSSNa membranes in a dual-chamber cell containing 5 mM NaCl solution and deionized water. I-V curves under a -2.0 V voltage (driving Na+ transport) show that the current for the GO membrane increases linearly from 0 to -2.4 μA, while the ArGO-PSSNa membrane current increases to -1.3 μA, indicating significant Na+ transport resistance in the ArGO-PSSNa membrane. Under positive voltage (driving Cl- transport), the GO and rGO membranes display currents rising to approximately 2.4 μA, but the ArGO-PSSNa membrane maintains a low current of around 0.1 μA, showing effective Cl- rejection.
Molecular dynamics (MD) simulations reveal that ArGO-PSSNa channels exhibit a higher energy barrier for ion transport compared to rGO channels. Both Na+ and Cl- can pass through rGO channels but not through ArGO-PSSNa channels. The mean square displacement (MSD) analysis shows that ion diffusion rates are significantly reduced in ArGO-PSSNa channels. Density distribution analysis confirms that Na+ accumulates around PSS within the nanochannels, implying strong electrostatic attraction between PSS and Na+. Radial distribution function (RDF) results indicate that rGO channels exhibit stronger Na+ dehydration effects than ArGO-PSSNa channels, suggesting that single-ion dehydration alone does not enhance salt rejection. DFT results demonstrate that strong electrostatic PSS-Na+ interactions are essential for ion confinement within nanochannels, contributing to high salt rejection.
In summary, the study’s strategy of electrostatically induced ion-restricted distribution in rGO nanochannels disrupts anion-cation coupling, significantly enhancing desalination performance. This approach presents a novel perspective for designing high-performance seawater desalination membranes and insights into ion transport mechanisms in GO membranes.