Wuhan University of Engineering: Graphene-Wrapped Porous Polyaniline/Manganese Oxide Nanocomposites for High-Performance Symmetric Supercapacitors
1. Research Summary
Manganese oxide has become an ideal electrode material for supercapacitors. Despite efforts to enhance the conductivity and structural stability of manganese oxide/conductor nanocomposites, achieving efficient and reliable energy storage electrode materials remains a challenge. In a study published in POLYMER COMPOSITES, Associate Professor Huang Huabo and Professor Ji Jiayou from Wuhan University of Engineering proposed a detailed “dual enhancement” strategy. This method synthesizes a three-dimensional (3D) nanostructured polyaniline/manganese oxide (PM) composite through an in situ process, which is further integrated with a graphene coating to form the polyaniline/manganese oxide/graphene nanocomposite (PMG).
Electrochemical analysis shows that the PMG composite has a specific capacitance as high as 403 F g⁻¹ (at 1 A g⁻¹) with a capacitance retention of 80.2% after 5000 cycles and a wide potential window. These enhancements are attributed to the synergistic effects of polyaniline and graphene, where polyaniline provides a supporting framework and electronic transport pathways, while graphene offers external protection and strengthens conductivity. The assembled symmetric supercapacitor demonstrates excellent energy density (32.7–23.3 Wh kg⁻¹) and power density (720–4500 W kg⁻¹) at a high working voltage of 1.8 V, surpassing the performance of many reported high-performance supercapacitors. This research provides valuable insights for advancing manganese oxide electrode materials, which could promote their widespread application in energy storage.
2. Visual Guide
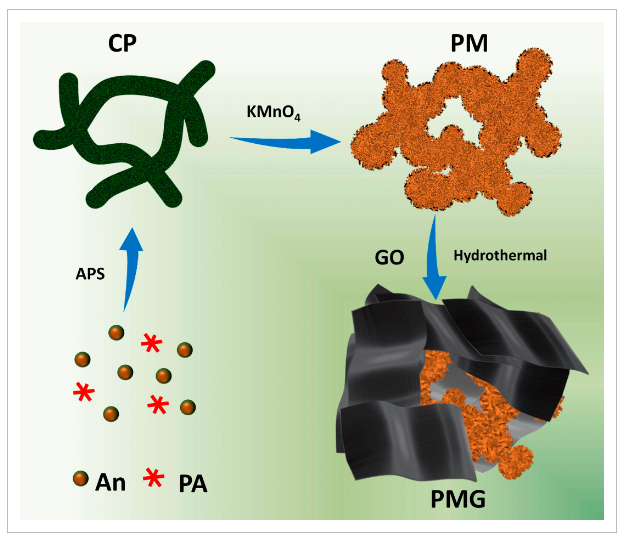
- Figure 1: Schematic diagram of PMG synthesis.
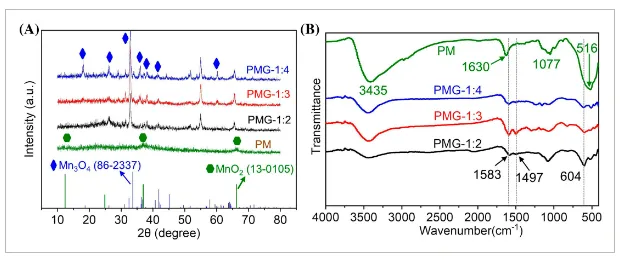
- Figure 2: XRD and FTIR spectra for PMG-1:2, PMG-1:3, PMG-1:4, and PM composites.

- Figure 3: SEM images of 3D-PANI (A), PM (B), and PMG-1:2 (C, D). TEM image of PMG (E) and HR-TEM image of manganese oxide region in PMG-1:2 (F). SEM image of PMG-1:2 (G) and elemental distribution maps of C (H), Mn (I), and O (J).
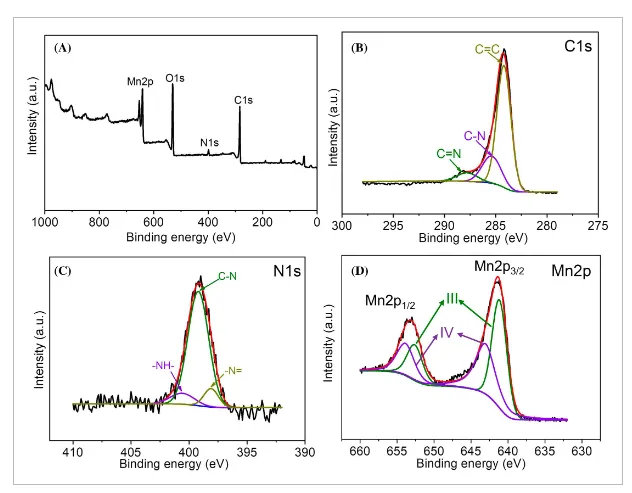
- Figure 4: XPS spectra of PMG-1:2 (A), with deconvoluted peaks of C1s (B), N1s (C), and Mn2p (D).
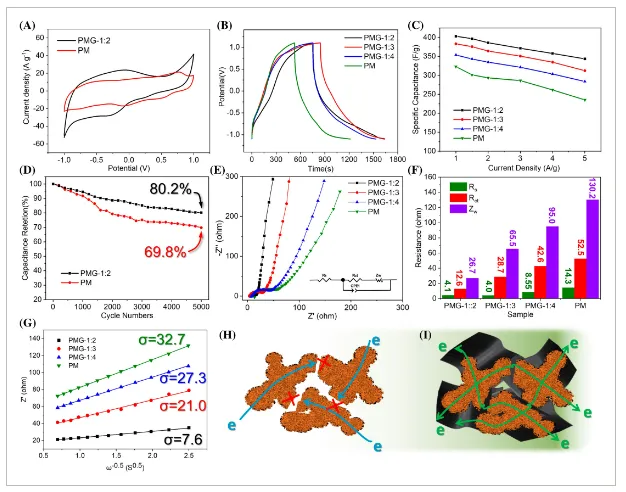
- Figure 5: Electrochemical performance of various electrode materials in a three-electrode system.
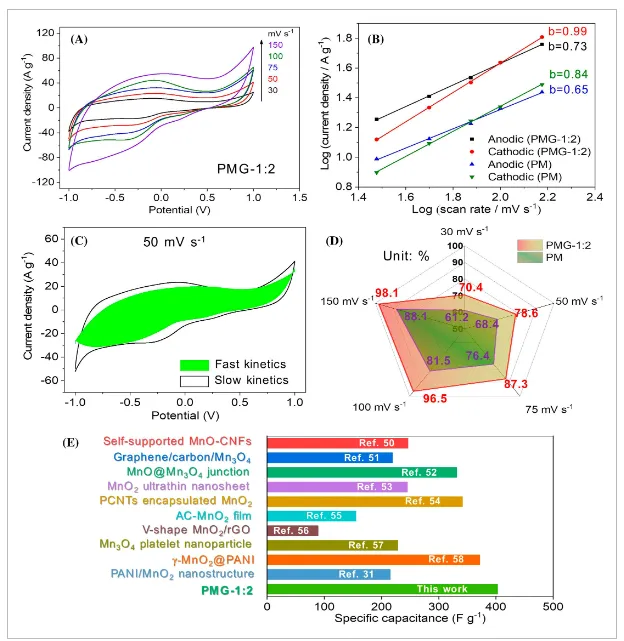
- Figure 6: Kinetic analysis.
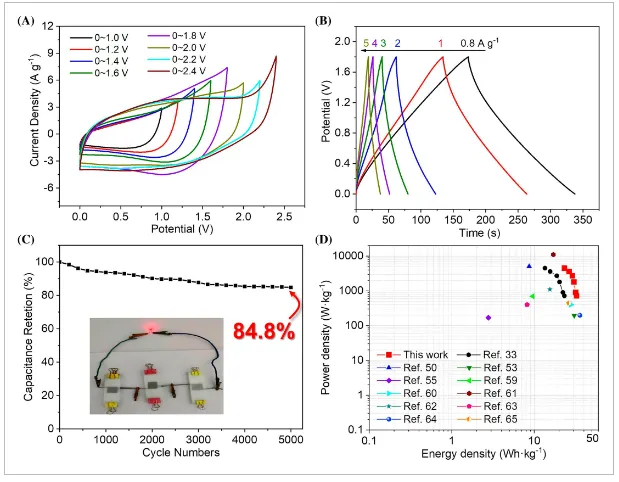
- Figure 7: Electrochemical performance of a symmetric supercapacitor assembled from two identical PMG-1:2 electrodes.
3. Conclusion
This study successfully developed 3D nanostructured polyaniline/manganese oxide/graphene (PMG) composites using the “dual enhancement” strategy and applied them to high-performance symmetric supercapacitors. The in situ formation and rGO encapsulation significantly enhanced the conductivity and structural stability of the PMG composite. The chemical composition, structure, and microstructure of the composite were comprehensively characterized using FTIR, XRD, XPS, SEM, and TEM. Electrochemical characteristics of PMG were studied using a three-electrode system. The results indicate that the specific capacitance of the PMG composite can reach up to 403 F g⁻¹ at 1 A g⁻¹, with a capacitance retention of 80.2% after 5000 cycles at 5 A g⁻¹.
Additionally, PMG exhibits a wide voltage window (-1 to 1 V), making it suitable for symmetric supercapacitor assembly. EIS and CV studies at different scan rates show that rGO encapsulation optimizes the electronic/ionic conduction and rapid electrochemical kinetics of the electrode materials. The assembled symmetric supercapacitor demonstrates superior energy and power densities at a wide operating voltage of 1.8 V. At 720 W kg⁻¹, the maximum energy density and power density reach 32.7 Wh kg⁻¹ and 4500 W kg⁻¹, respectively, outperforming many related symmetric and asymmetric supercapacitors. This research offers a comprehensive material design strategy, providing new possibilities for improving supercapacitor performance and expanding their application areas, with potential further applications in other electrochemical energy storage materials and devices.

